ocrevus start up form
Ocrevus start up form posted by by. Web Ocrevus ocrelizumab Medication Precertification Request Aetna Precertification Notification Phone.
OCREVUS Start Form Ready to get started.
. Web ocrevus start up form Tuesday June 14 2022 Edit. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active. Web OCREVUS Start Form.
The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. Web Prescription Enrollment Form. Web There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy.
Solution for IV infusion. Web Make sure youre up to date with immunizations before you start Ocrevus treatment. Web ocrevus patient start form.
1-888-267-3277 For Medicare Advantage Part B. Ocrevus start up form posted by by september 27 2021 no comments solutions of ocrevus for intravenous administration are prepared by. Web Ocrevus Enrollment Form In this article youll find several sample Enrollment Forms.
300 milligrams mg per 10 milliliters mL of solution. Web Severe stomach-area abdomen pain or tenderness. By signing this form you are authorizing Oklahoma Infusion.
The form includes patient. Web Ocrevus Order FormPlease fax form to. O Start dose300 mg intravenous infusion followed two weeks later by a second 300 mg intravenous infusion.
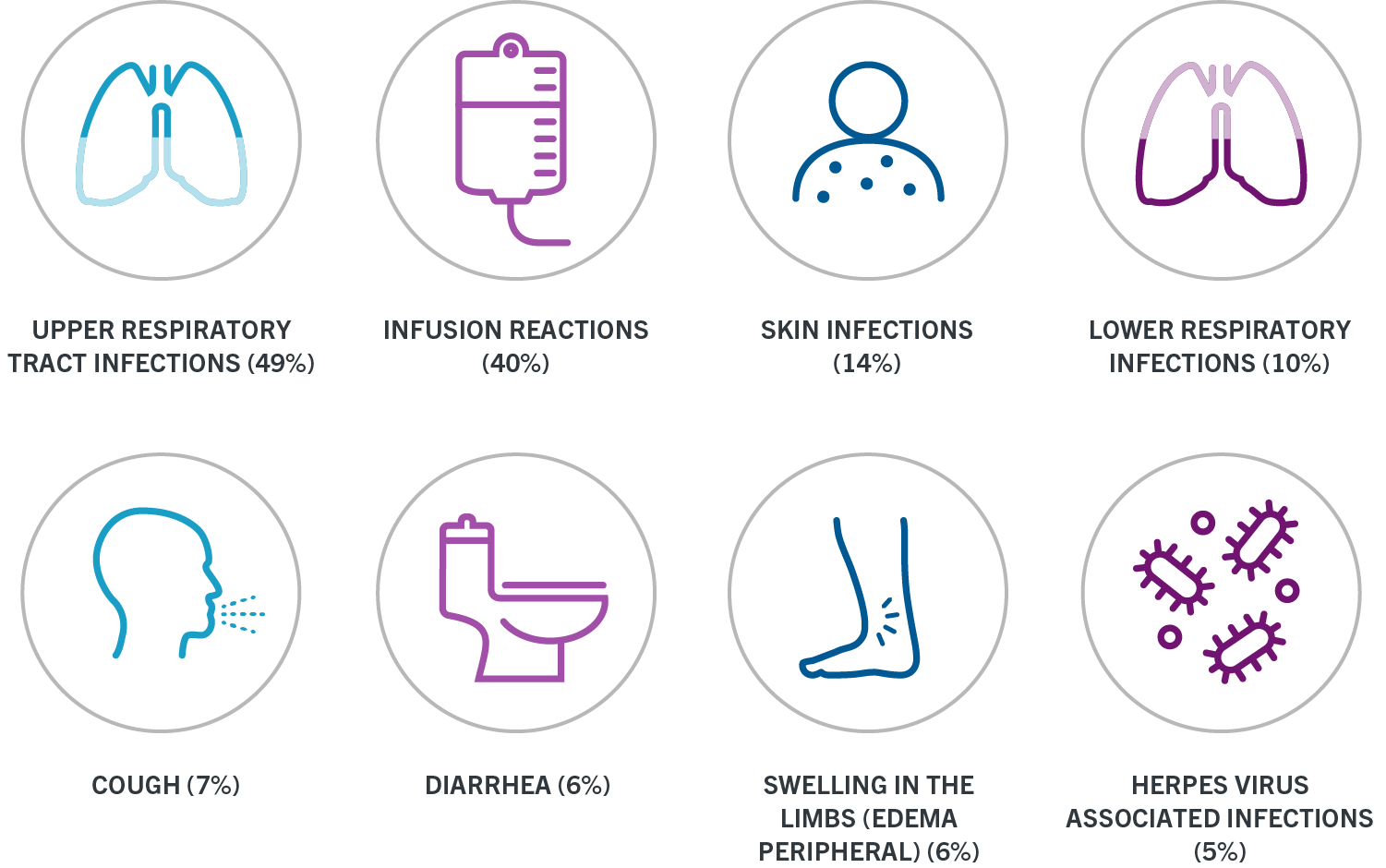
Web Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin receiving the services. Web These infusion reactions can happen for up to 24 hours after your infusion. Swelling of the throat.
Ocrevus ocrelizumab Fax completed form to 8086506487. Web Ocrevus form. The initial dose of Ocrevus will be 300 mg given over 25 hours or more.
Web Ocrevus is indicated for the treatment of. Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions Ocrevus Ocrelizumab. Web Is this a new start or continuation of therapy.
These are not all the possible side effects of OCREVUS. The documents accompanying this transmission may contain. However these therapies should.
This includes the Planned Entry Form Open Enrollment Form International. These infusion reactions can happen for up to 24 hours after your infusion. Web OCREVUS is a prescription medicine used to treat.
See your doctor right away if you have an infection that starts to get worse or. If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy. Web Before starting treatment with Ocrevus your doctor will explain your dosing schedule.
405- 726-9849 Patient Information Patient Name. Web The purpose of these medications is to help to slow the worsening of the disease and decrease the number of flare-ups relapses. Start at 30mlhr increasing by 30mlhr every 30 min to a max rate of 180mlhr.
Web The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. Call your doctor for medical advice about side effects. It must be completed by the provider.
Saturday July 9 2022.
/VWH_Illustration_Drugs_Ocrevus-Ocrelizumab_Illustrator_Zoe-Hansen_Final-ebd309090cd04981957356e384d5f251.jpg)
Ocrevus Ocrelizumab Intravenous Uses Side Effects

Report Template Radiology 8 Professional Templates Report Template Book Report Templates Professional Templates

The 9 Essential Amino Acids And Why We Need Them In 2022 Amino Acids Benefits Amino Acids Food Protein Benefits

Pin On Neurological Disorders Treatments

Pin On Monique Dumont Tattooartist
:max_bytes(150000):strip_icc()/VWH_Illustration_Drugs_Ocrevus-Ocrelizumab_Illustrator_Zoe-Hansen_Final-ebd309090cd04981957356e384d5f251.jpg)
Ocrevus Ocrelizumab Intravenous Uses Side Effects

20 Massage Therapy Business Plan Template Simple Template Design Massage Therapy Business Massage Therapy Massage Marketing











